OUR SERVICE
Studies that require a sponsor always must have sufficient funding to cover all associated costs.
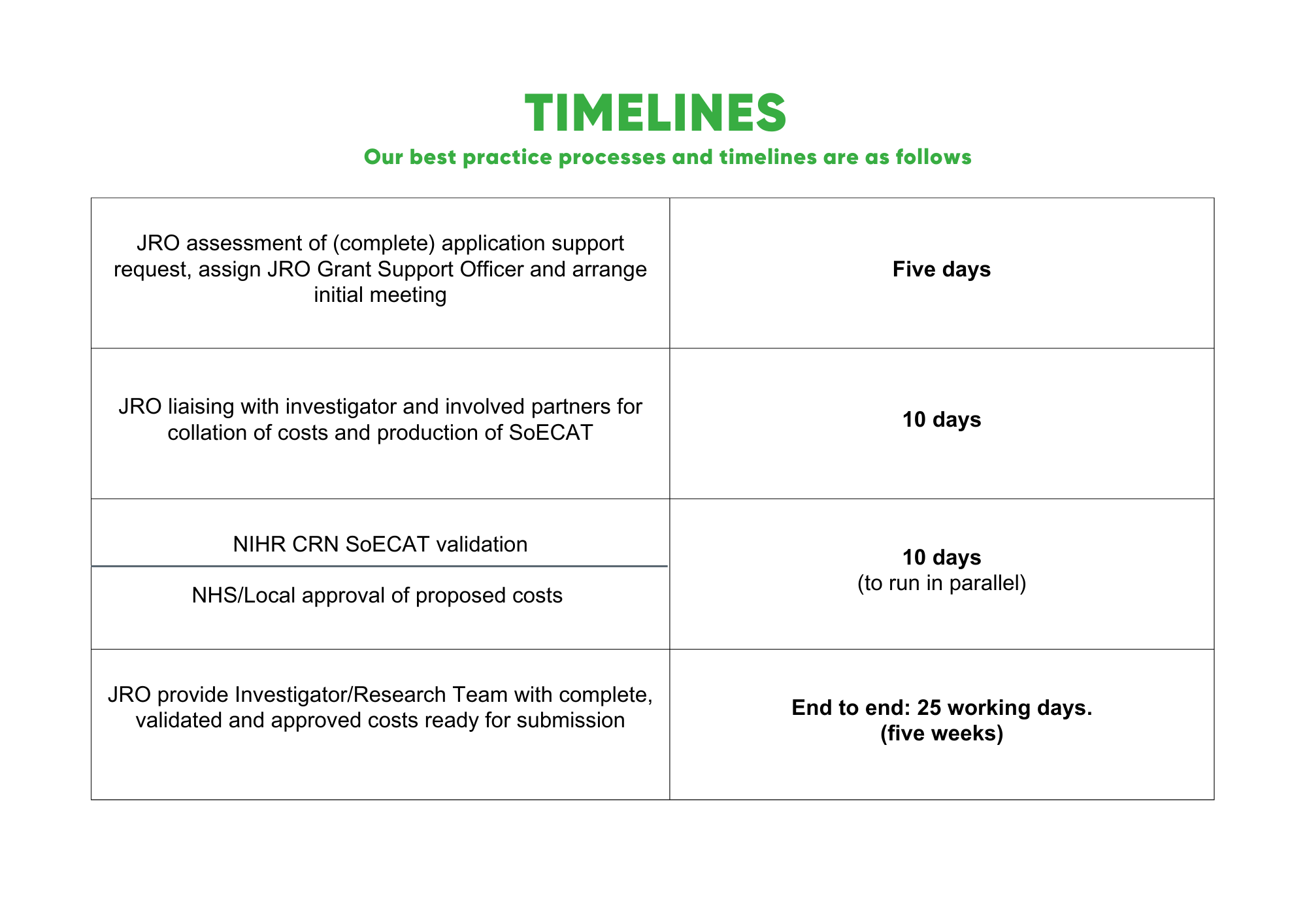
Grant applications for clinical research taking place at any of our partner organisations should be reviewed and supported by the JRO before their submission. The JRO grants team can guide investigators through the process, convening partners and collaborators, ensuring all costs are accurately calculated, approved and submitted. The team will advise and support completion of the SoECAT for studies requiring this from the funder.
The grants team will ensure all approvals are received timely prior to submission.
There is a request for timeframes to be adhered to when working with the JRO grants team.